
NGS 业界在 2020 年与 COVID-19 的抗争中见证了令人难以置信的协作。研究者们就冠状病毒发表了成千上万篇经同行评审的手稿,同时有超 45,000 个序列提交给 GenBank。Twist Bioscience 推出了 SARS-CoV-2 合成 RNA 质控品和 NGS 靶向富集组合,用于在 COVID-19 患者中进行病毒检测。Oxford Nanopore、MGI 和 Illumina 在测序技术方面取得了重大进展,其中包括提高了准确性和效率,降低了研究人员的成本。凭借全外显子组测序,第一个探索突破性逆转 1 型糖尿病的案例研究得以发表。充满变故的 2020 年即将翻过终章,以下是年度亮点回顾:
令人难以置信的动员寻得战胜 COVID-19 疫情的方法
Researchers around the world are collaborating in extraordinary ways to tackle the COVID-19 pandemic. Roughly 4% of the world’s research output was focused on the coronavirus, with over 100,000 published articles. The number of sequences submitted to GenBank grew exponentially, with over 45,000 submitted sequences. At the beginning of the pandemic, papers focused on the spread, outcomes, diagnostics, and testing. As the year went on, the shift focused to mental health research related to the virus.
In addition, over 300 COVID-19 in vitro diagnostic kits have had Emergency Use Authorization (EUA) approvals, spanning over 9 kinds of technology including antigen lateral flow, real-time reverse transcriptase polymerase chain reaction (qRT-PCR), electrochemiluminescence immunoassays (ECLIAs), enzyme linked immunosorbent assays (ELISAs), and many more. EUAs allow the Food and Drug Administration (FDA) to strengthen public health protection against threats by facilitating the availability of medical countermeasures (MCMs) needed during emergencies.
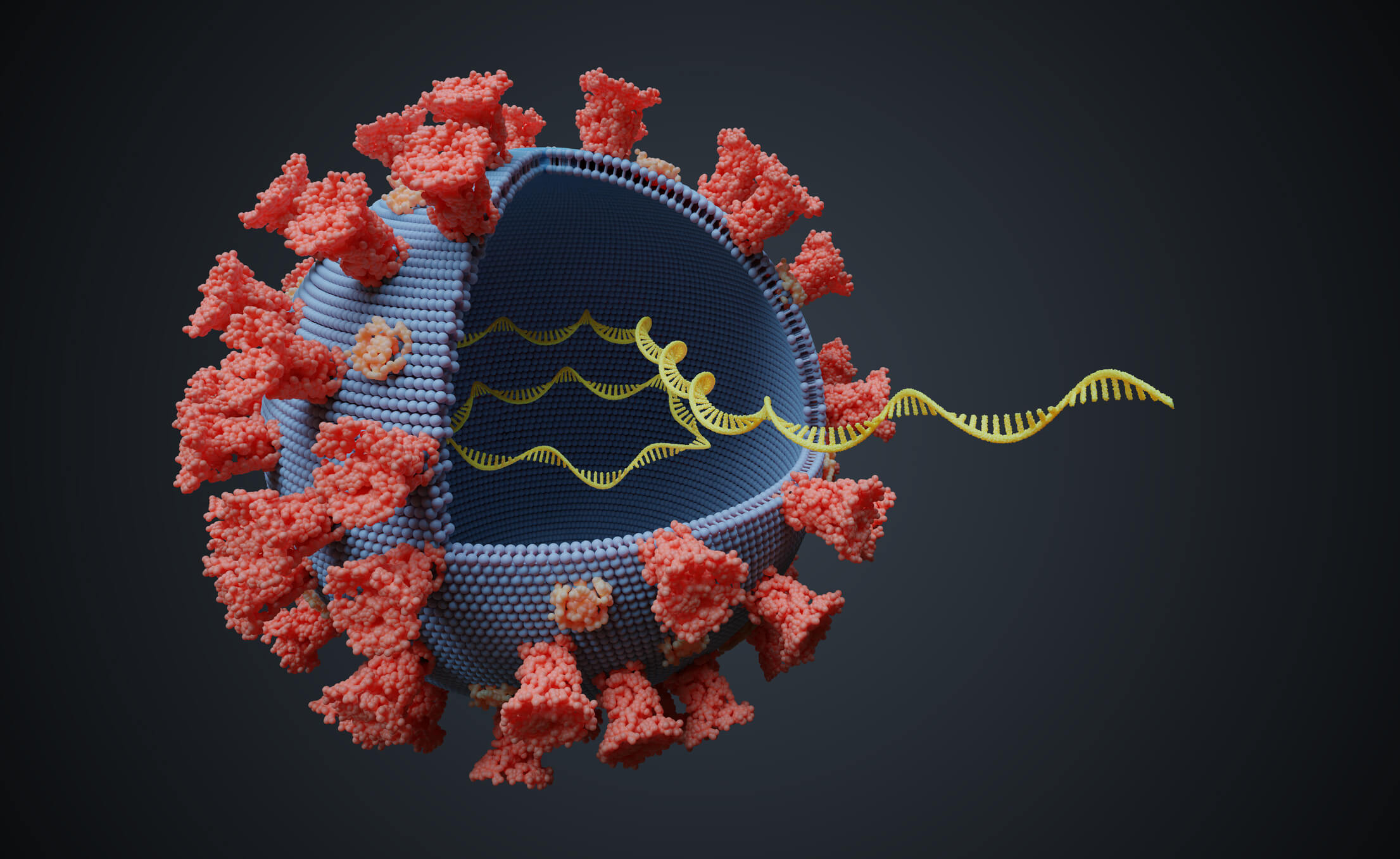
Twist Bioscience 推出了用于 COVID-19 检测的合成质控品
On March 12th, 2020, Twist Bioscience announced the availability of two synthetic SARS-CoV-2 RNA controls. These controls provide quality control for the development, verification, and validation of diagnostic tests including next-generation sequencing (NGS) and RT-PCR for COVID-19. The two controls cover the full SARS-CoV-2 viral genome and are sequence-verified. The FDA added the Twist controls as reference materials to their website on March 25th, 2020.
在宣布了两个合成 SARS-CoV-2 RNA 质控品后仅四天,Twist Bioscience 宣布推出 NGS 靶向富集组合,用于病毒检测和鉴别 SARS-CoV-2 阳性检测的患者。这些组合旨在用于环境监控和监督测试,同时提供对序列信息的洞察,以跟踪病毒的进化。通过使用其专有的硅基 DNA 合成平台,以及使用以 DNA 为基础的杂交探针进行靶向富集,Twist Bioscience NGS 组合可从血液、鼻腔和粪便样本中鉴定高通量和特异性感染。这些靶向富集工具用于专门检测纽约市地铁上的 SARS-CoV-2, 同时过滤掉其他遗传物质,从而获得了更具成本效益和可靠的测序结果。
为了快速应对 12 月在英国和南非出现的新的、更易传播的菌株,Twist Bioscience 正在利用其平台合成技术和基础设施,提供额外的质控品来检测这种特定变体。
Oxford Nanopore 的 PromethION 准确率达到 99.1%
During the 2020 Nanopore Community Meeting earlier this month, Oxford Nanopore revealed a few key advances in sequencing technology. First, their large-scale, long-read, direct DNA and RNA sequencing platform, PromethION, reached a record 99.1% modal single-read accuracy using improved sequencing chemistry, high-accuracy variant calling tools, and automation options at scale. Second, updates to their new analysis algorithm, Bonito CRF, have shown 98.3% single-read accuracy, which is corroborated by customer reports. These updates also improved structural variation accuracy to 96% with only 30X coverage. Single nucleotide polymorphism accuracy also reached 99.92%. Third, Oxford Nanopore collaborated with Hamilton and Opentrons to provide automation solutions for high-throughput projects or everyday workflows such as library preparation or liquid handling.
MGI 和 Illumina 提供平价的测序平台
In February during the Advances in Genome Biology and Technology annual meeting, MGI announced the DNBSeq Tx “extreme throughput” sequencing platform, able to produce human genomes for only $100 in consumables costs. This platform consists of several components that spatially uncouples fluidics from imaging and uses large open slides instead of flow cells to carry the DNA nanoball arrays. It has an output of 70 terabases per run and has a runtime of 3.5 days and can process up to eight independent slides, each of which can produce data for up to 150 human genomes at 30X coverage. However, there are some caveats. In the US, the DNBSeq Tx platform will only run with the antibody-based CoolMPS sequencing chemistry. MGI has also not determined a list price. Lastly, MGI is currently in litigation disputes with Illumina, who was granted preliminary injunction for two patent lawsuits.
In August, Illumina announced the NovaSeqTM 6000 v1.5 Reagent Kit, making whole-genome sequencing more accessible and affordable. With only a $600 price tag, labs can now perform single-cell genomics, whole-genome sequencing, and liquid biopsy with flexibility and accuracy at scale. This kit has been enhanced to include improved quality specification score (Q30 scores upwards of 85% for read lengths up to 2x150bp), fully supported unique molecular identifiers, extended shelf life (six months instead of the previous three months), and support for new library prep kits and assays such as the Illumina DNA PCR-Free Prep Kit.
Illumina 以 80 亿美元收购 GRAIL
After filing to go public, GRAIL, a healthcare company focused on early cancer detection, was acquired by Illumina for $8 billion in cash and stock. This acquisition adds a multidisciplinary team to address NGS, population-scale clinical studies, and machine learning to multi-cancer screening, with earlier detection and better outcomes. GRAIL has been making progress on its GalleriTM multi-cancer screening test using methylation signatures in cell-free DNA. This technology will be used for broadly available blood-based cancer screening that will make detection more effective and cheaper. GalleriTM is expected to launch in 2021.
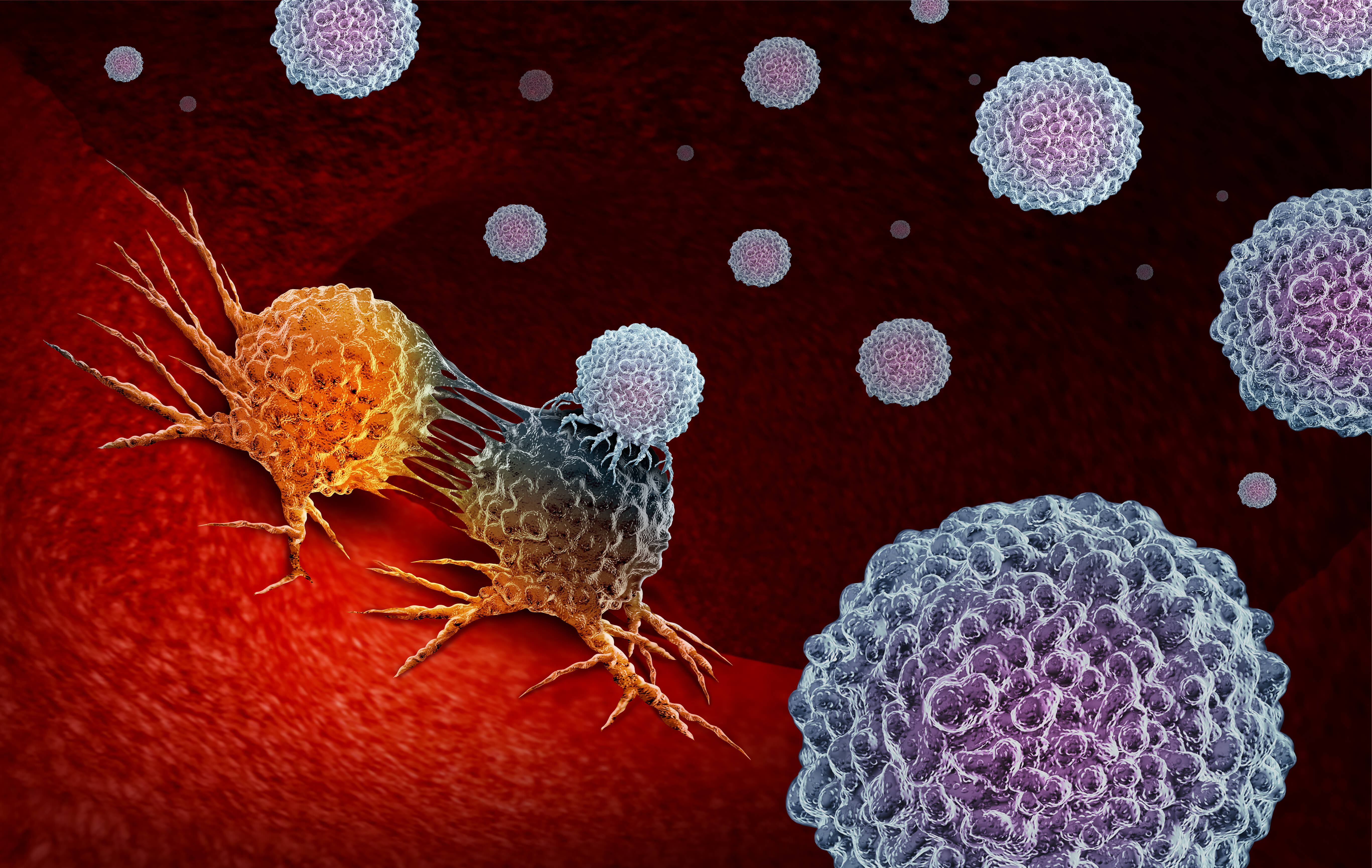
Guardant Health 和 Foundation Medicine 因液体活检技术而陷入法律之争
Guardant Health, a leading precision oncology company, received FDA approval for its Guardant360® CDx, a liquid biopsy and NGS platform that uses high-throughput tumor profiling on blood to provide genetic information about a patient’s tumor in just seven days. The approval is for identifying epidermal growth factor receptor (EGFR) mutations in patients who might benefit from TAGRISSO (osimertinib), an FDA- approved therapy for metastatic non-small cell lung cancer (NSCLC). This less-invasive method for comprehensive genomic profiling moves beyond limitations of tissue specimens and provides better assessment of tumor composition, making it easier to identify problematic mutations in 55 tumor genes simultaneously.
In November, Guardant filed a lawsuit against Foundation Medicine, owned by Roche, for infringing on seven patents related to liquid biopsy. Foundation received FDA approval for its FoundationOne® Liquid CDx pan-tumor liquid biopsy test in August. This platform also uses a simple blood draw to analyze over 300 genes and genomic signatures for all solid tumor cancers. Because of the COVID-19 pandemic, the patent infringement jury trial will be on hold until March 2021.
外显子组测序精密医学逆转 1 型糖尿病
In their correspondence to the editor of The New England Journal of Medicine, Chaimowitz et al. describe a case study of a 17-year-old boy with type 1 diabetes (T1DM). Whole-exome sequencing revealed a pathogenic heterozygous gain-of-function mutation in STAT1, a transcriptional activator involved in cell survival or pathogen response. STAT1 核输出可以由 JAK1 激酶活性进行调节。该患者接受了 JAK1/2 抑制剂鲁索替尼的治疗,该药物能够逆转其 T1DM。该患者能够降低胰岛素剂量,并最终在开始使用鲁索替尼后一年,被被诊断出 T1DM 后近两年时,完全停止了剂量。
With all of these new technologies in NGS diagnostics and treatment, the NGS industry will be riding a wave of momentum going into 2021! Twist is proud to support the scientific community with robust tools for NGS analysis.
您怎么认为?
喜欢
不喜欢
喜爱
惊讶
有兴趣
